Translate this page into:
Hot orbits of dental origin – Case series with review of literature

*Corresponding author: Dayna Wei Wei Yong, Department of Ophthalmology, National University Hospital, Singapore, Singapore. daynayong6@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: De Barros AS, Yong DW, Sundar G. Hot orbits of dental origin – Case series with review of literature. Lat Am J Ophthalmol. 2025;8:2. doi: 10.25259/LAJO_26_2024
Abstract
The cases illustrated uncommon cancers that presented as orbital cellulitis following dental treatments. Recognizing orbital tumors was essential for the patients’ prognosis. The relevance of differential diagnosis in situations involving periorbital swelling is underscored by these unique cases. The patients in Case 1 and Case 2 showed periorbital swelling, proptosis, and ocular pain. Consequently, treatment for orbital cellulitis was initiated given the recent history of dental procedures. Nevertheless, imaging studies and a biopsy were requested for additional investigation due to inadequate therapeutic response. In Case 1, a secondary orbital lymphoma originating from the maxillary sinus is discussed. Thus, the treatment strategy involved adjuvant radiotherapy and chemotherapy. In Case 2, a rare rhabdomyosarcoma is observed in an 18-year-old patient. The patient is still undergoing chemotherapy as part of the therapeutic plan. Therefore, the subsequent assessment changes the initial diagnosis of orbital cellulitis. Case 1 and Case 2 demonstrate the relevance of observing the clinical progress and treatment outcome of patients with periorbital swelling following dental procedures. In this way, this approach can help identify malignancy, preventing severe complications.
Keywords
Dental procedure
Orbital cellulitis
Orbital tumor
Rhabdomyosarcoma
Diffuse large B-cell lymphoma
INTRODUCTION
Odontogenic orbital cellulitis has been well-reported. Not all patients with periorbital swelling following dental procedures have orbital cellulitis. In fact, sinister underlying pathologies are not uncommon. The analysis of the two cases presented shows that periorbital swelling due to dental procedures is a pathology of vision and the maxillary sinus, which ophthalmologists must be aware of. The patients in both cases consented to the use of clinical photographs for archiving, management, and publication. There are no conflicts of interest to report.
CASE 1
A 43-year-old East-Asian male presented with left eye (LE) and facial swelling. Two days following a left root canal procedure. He was previously reported well otherwise. At the presentation, he was afebrile, alert but was in pain. On examination, best-corrected visual acuity (BCVA) in the right eye (RE) and LE were 6/6 and 6/24, respectively. Intraocular pressures (IOP) were 19 mmHg bilaterally.
Pupils were equal and reactive to light (PEARL) with no relative afferent pupillary defect (RAPD). Ishihara was full and confrontational visual fields were normal. There was LE adduction and infraduction limitation. The left conjunctiva was hyperemic with chemosis. The rest of the anterior segment was normal. Fundus examination, including optic nerve head examination, was unremarkable. Periorbital examination revealed numbness over the left V2 distribution. The working diagnosis was left orbital cellulitis and orbitofacial imaging was performed. A contrast-enhanced computed tomography scan revealed a large heterogeneous collection containing gas centered around the left maxillary sinus, molar region, and ethmoid sinus, which were suspicious for an infected hematoma. There was left intraorbital extension with displacement and thickening of the inferior rectus, mild lateral displacement of the left medial rectus and mild proptosis. There was expansion and bony destruction of the left maxillary sinus walls [Figure 1].
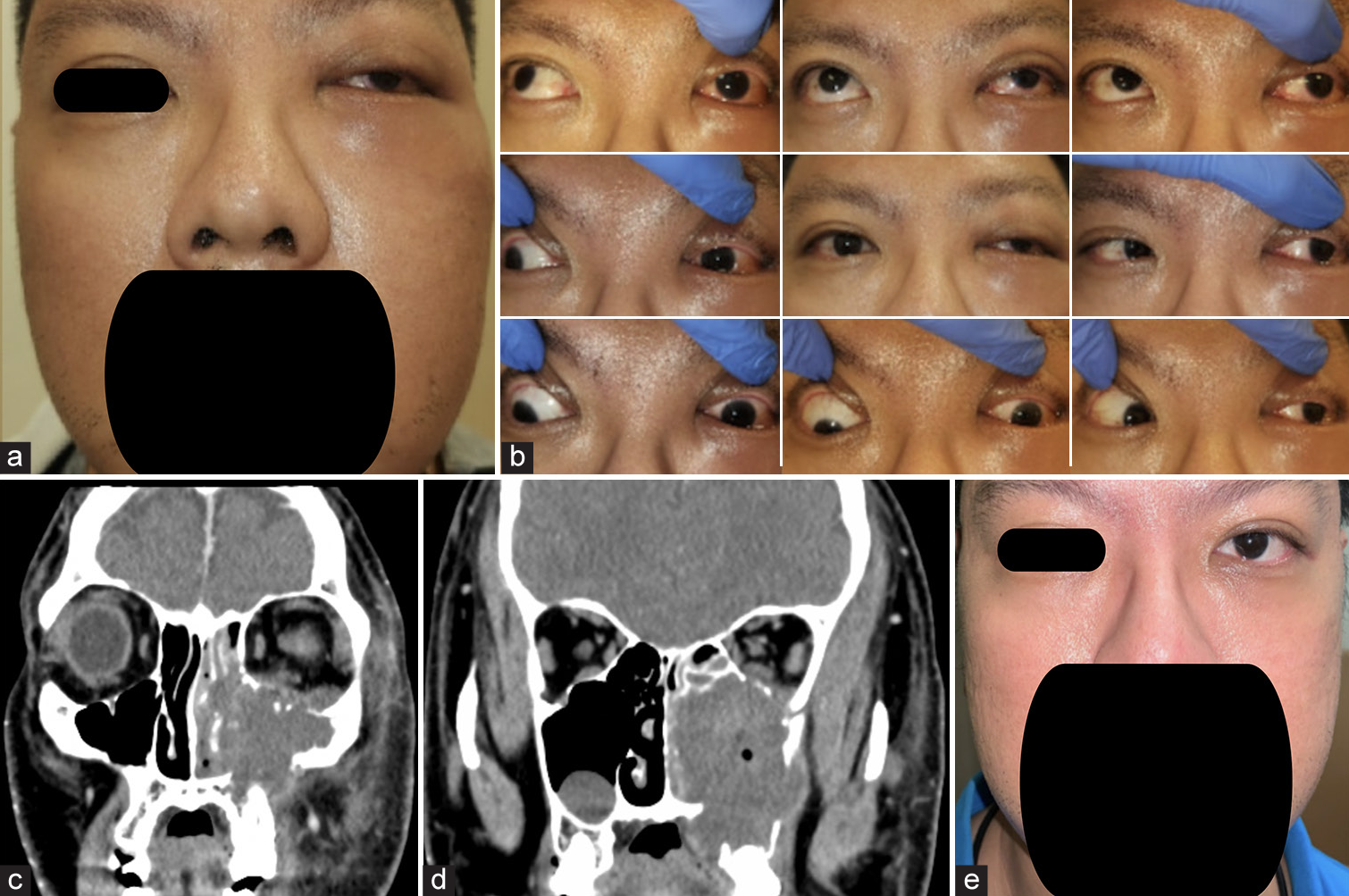
- (a) Left eye (LE) hypoglobus, fullness of left lower lid with cheek swelling. (b) 9-gaze photo shows limitation of LE on adduction and depression. (c) Computed tomography (CT) face non-contrasted scan, coronal cuts, revealed a large heterogeneous gaseous collection at left maxillary sinus and upper molar region, extending into ethmoid sinus and orbit; bony erosion of maxillary sinus walls and orbital floor. (d) CT face non-contrasted scan, coronal cuts, revealed a large heterogeneous gaseous collection at left maxillary sinus and upper molar region, extending into ethmoid sinus and orbit; bony erosion of maxillary sinus walls and orbital floor. (e) Clinical image lymphoma treatment (2 weeks from presentation).
An Ear, Nose, and Throat (ENT) consultation was obtained, and a functional endoscopic sinus surgery was performed. Intraoperative findings were as follows: congested idle and inferior meatus, with maxillary sinus filled with blood clots, fibrinous material, and turbid brownish fluid. No masses/cysts were noted within the maxillary sinus. Intraoperative cultures were obtained and specimens were sent for histopathology. Microbiological cultures grew Staphylococcus lugdunensis and the patient was started on intravenous augmentin (changed to oral metronidazole 5 days later) with significant clinical improvement. Histopathologic examination, however revealed a diffuse large b-cell lymphoma (DLBCL) (non-germinal subtype). Systemic workup revealed a final staging of limited-stage DLBCL [Figure 2]. He underwent R-CHOP R-CHOP (Cyclophosphamide, Hydroxydaunorubicin [Doxorubicin Hydrochloride], Vincristine Sulfate, and Prednisone) chemotherapy and 15 sessions of 30 Gy consolidation radiotherapy to head with almost complete return to normal. A repeat biopsy of left maxillary sinus performed 7 months post-diagnosis showed no evidence of lymphoma. At last follow-up 1.5 years later, the patient is doing well with full ocular motility and is in good clinical remission although BCVA in the LE was 6/21.
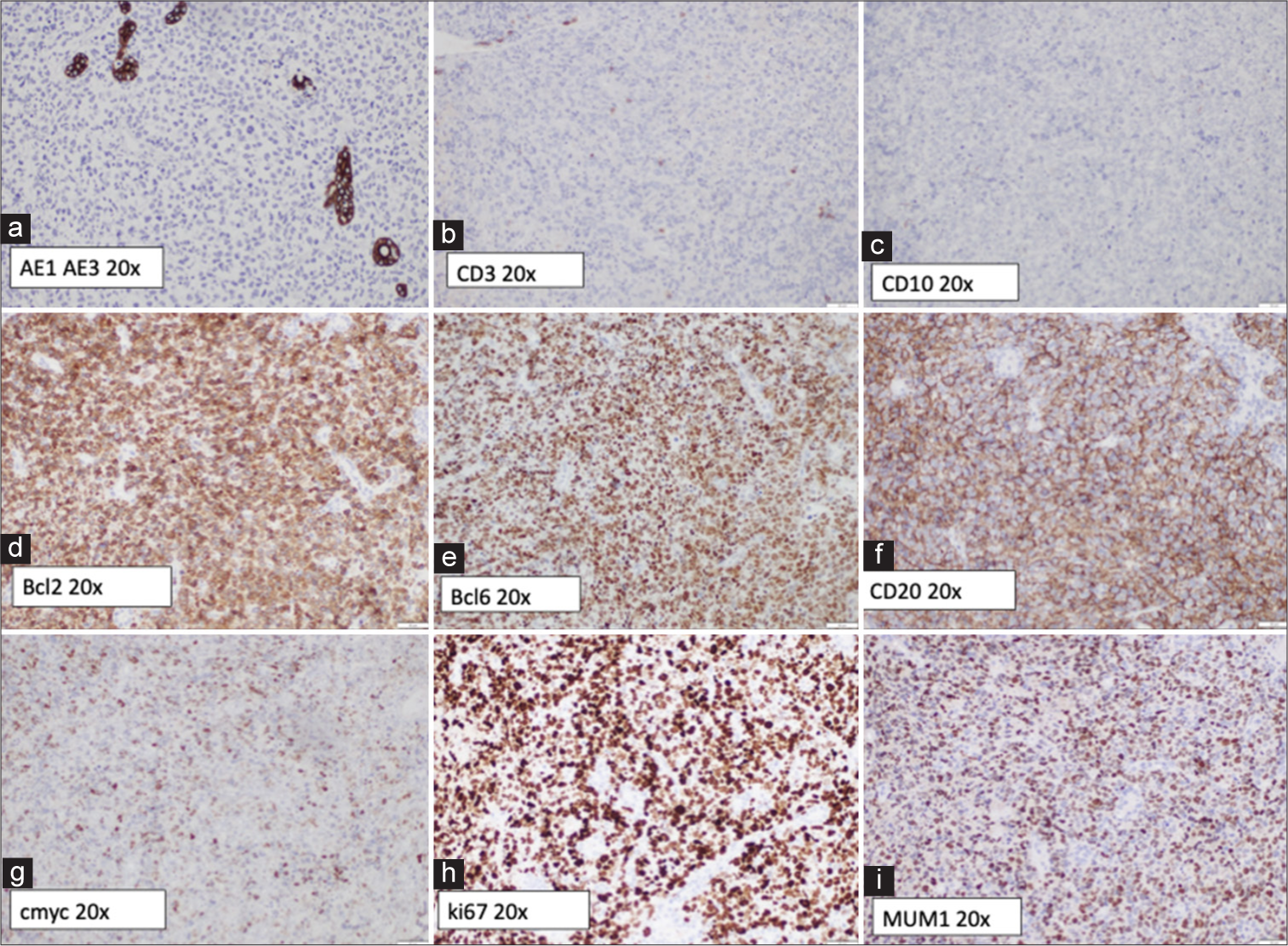
- (a) Tumor cells negative for anticytokeratin monoclonal antibodies (AE1 and AE3), excluding a carcinoma. (b) Tumor cells negative for CD3. (c) Tumor cells negative for CD10. (d) Tumor cells positive for BCL2 (>70%). (e) Tumor cells negative for B-cell lymphoma (BCL6). (f) Tumor cells staining positive for CD20. (g) About 40-50% of the tumor cells are weakly positive for c-myc. (h) The Ki-67 proliferative index is variable, reaching up to 50% in some areas, which could be due to suboptimal tissue preservation. (i) Tumor cells positive for MUM1 (Multiple Myeloma Oncogene 1).
CASE 2
An 18-year-old East-Asian male presented 2 months following a right upper and lower wisdom teeth extraction with protrusion of the RE with double vision. Review of systemic symptoms was unremarkable. On ophthalmic examination, his BCVA was 6/9 and 6/7.5 in the RE and LE, respectively. IOP was 15 mmHg on the RE and 14 mmHg on the LE. On examination, there was right facial swelling and proptosis. The pupils were equal in size and reactive to light, and there was no relative afferent pupillary defect. Optic nerve function was preserved. There was RE moderate abduction, adduction supraduction, and infraduction deficit. Anterior and posterior segment examinations were normal. Exophthalmometer periorbital examination revealed numbness over the right V2 region [Figure 3]. Initial clinical suspicion was right odontogenic orbital cellulitis and an orbitofacial imaging was performed. A magnetic resonance imaging (MRI) scan of the orbits and nasal cavity revealed a transpatial sinonasal/orbital/maxillary mass that was concerning for a high grade neoplasm. An urgent biopsy of the right sino-nasal-orbital tumor was performed endoscopic transnasally and confirmed rhabdomyosarcoma (RMS)-alveolar subtype [Figure 4]. He was referred to an oncologist and systemic workup revealed Stage 4 (T4N1M0) right maxillary alveolar RMS (ARMS). Following which he underwent vincristine sulfate, dactinomycin (actinomycin-D), and cyclophosphamide chemotherapy and completed a cycle of proton therapy in between. At 1 month follow-up, right proptosis improved with commencement of chemotherapy. He is still in the midst of chemotherapy.
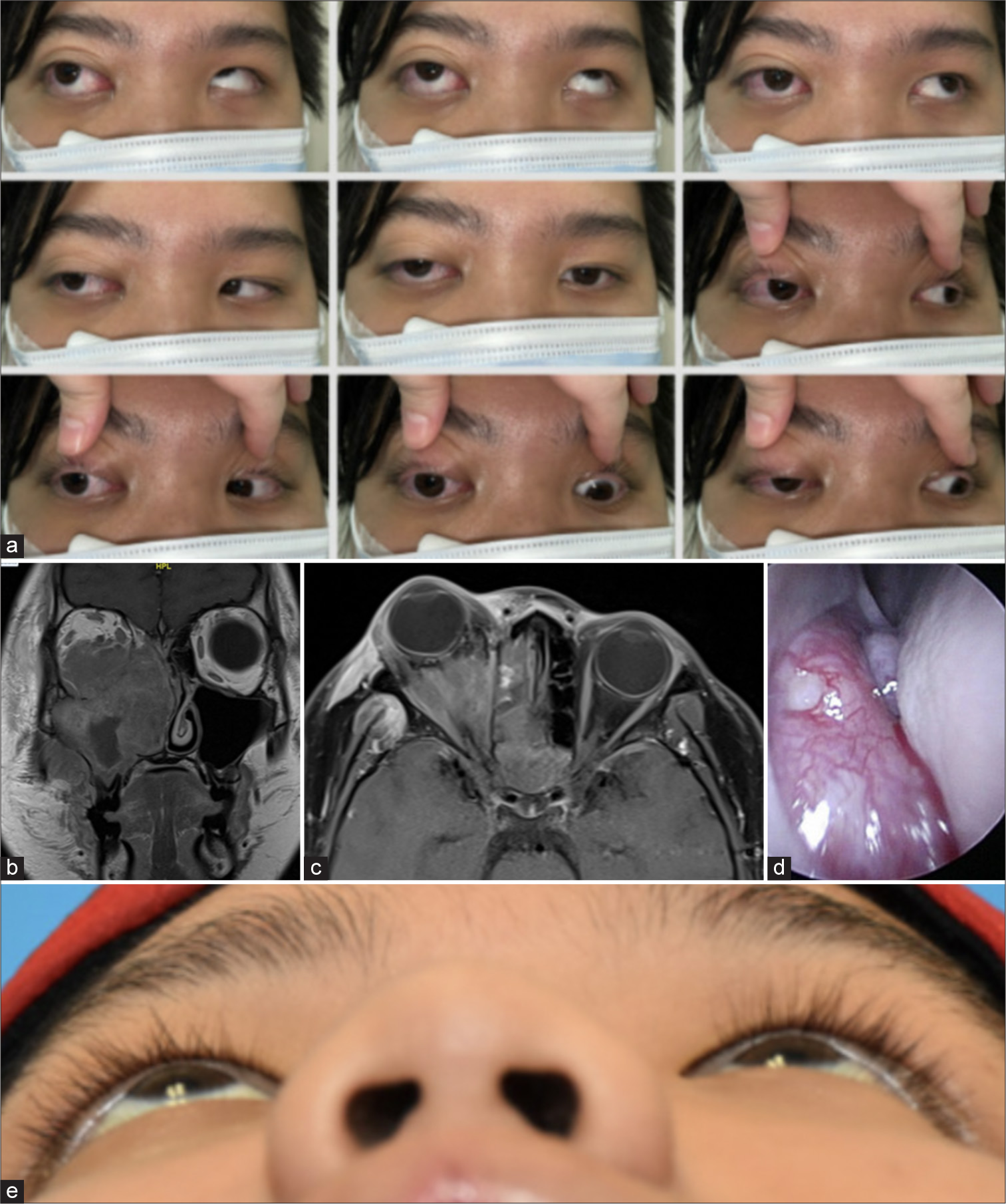
- (a) 9-gaze photo shows limitation of left eye in all directions. (b and c) Magnetic resonance imaging of the orbits (with contrast) coronal and axial cuts– hyperintense mass of right maxillary sinus extending into lateral nasal wall and ethmoidal spaces, with irregular infiltration or bony erosion. The mass invades the medial, inferior, and lateral recti muscles. (d) Nasoendoscopy revealed a fleshy mass at middle and inferior meatus. (e) Worms eye view demonstrating improvement in proptosis.
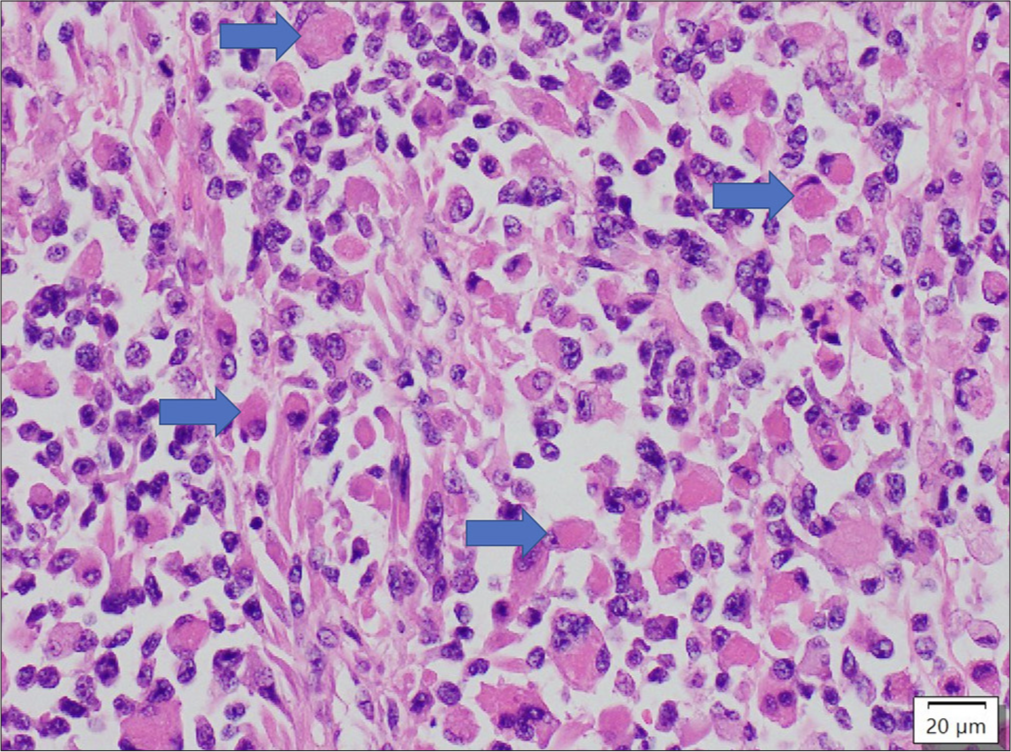
- Sheets of rhabdoid cells with eosinophilic cytoplasm. The blue arrows depict some of the nuclei of the tumour cells were being pushed to one side. 400x, haematoxylin and eosin stain
DISCUSSION
Periorbital swelling following dental procedures can occur because odontogenic infections spread to adjacent tissues.[1] Orbital cellulitis involves inflammation of the orbital and periorbital fat, extraocular muscles, and other soft tissues.[1,2] If left untreated with antibiotics, this infection is a dangerous illness that can potentially lead to blindness or even death due to cavernous sinus infection and thrombosis.[1,2] Early signs, including eyelid redness and edema, are present in more than 70% of cases.[1] However, symptoms such as conjunctival chemosis, ptosis, periocular pain, eye movement pain, and fever may require additional tests due to increased severity.[1,3]
Prompt imaging of the orbits, face, and brain, if needed, is essential for serious signs.[1,2] This can be done either with a CT scan, as in Case 1, or with an MRI, as in Case 2. These modalities typically reveal non-specific findings, such as opacification of the paranasal sinuses and dehiscence of the floor of the maxillary sinus.[1,2] Additional findings, including expansion and destruction of the maxillary sinus roof/orbital floor and soft-tissue density, suggest severe conditions, like malignant tumors.[4,5] Regardless, sinus disease progression might cause intraorbital involvement, leading to visual compromise and thrombosis of the orbital veins and cavernous sinus.[2,4,5]
In both cases, initial diagnosis of orbital cellulitis was considered, given pain, proptosis, ocular motility limitation, and periorbital edema following a dental procedure. However, with imaging followed by biopsy based on high clinical suspicion, this hypothesis changed. There is the possibility of orbital or sino-orbital malignancies when periorbital inflammation persists despite antibiotic treatment, or in the presence of unusual signs such as ongoing vision, progressive proptosis, or unusual features on orbito-facial imaging.[3,4]
In case 1, an important instance of secondary orbital lymphoma is noted, with about 42% of DLBCL cases arising as secondary orbital manifestations.[6,7] In the patient presented, the orbital pathology emerged from within the maxillary sinus, a form of sinonasal lymphoma, in which DLBCL and extranodal natural killer/T-cell lymphoma are the most frequent.[6] Orbital cellulitis was initially suspected, considering the patient’s age and recent history of a dental procedu-re.[1] The average age of patients with sinonasal DLBCL is between the fifth and sixth decades of life.[6] The extension of the lymphoma to adjacent structures, like the orbit, may cause proptosis and, in some situations, periorbital swelling is present.[6,7] In a large multicenter study conducted at international eye cancer centers (n = 779), when discussing primary orbital lymphomas, extranodal marginal zone B-cell lymphoma was the most frequent subtype (57%), followed by DLBCL (15%).[7]
In Case 2, a rare RMS was discovered. RMS is the most common orbital malignancy in the pediatric population, accounting for approximately 5% of the orbital masses.[5] The typical age of diagnosis is 6–8 years old, although it may present at birth.[8] Our case is unusual, as the patient presented at 18 years of age. The head-and-neck region accounts for 40–70% of all RMS cases.[5,9] Primary orbital RMS presents with rapid-onset proptosis, restriction, and painful ocular movements, similar to orbital cellulitis.[5,9] Histopathological types include embryonal RMS (ERMS), pleomorphic RMS, and ARMS. ERMS and ARMS typically occur in young children.[5,9]
Adult-onset RMS is an aggressive tumor that can appear in the sino-nasal-orbital region.[5] Its tendency to destroy bone highlights its intensity and fast growing, as demonstrated in our case by the hyperintense mass of the right maxillary sinus extending into the lateral nasal walls and ethmoidal spaces.[5,9] Accordingly, the diagnosis of ARMS is important to prevent the disease from spreading and threatening the patient’s vision.[9] Biopsy may reveal infiltration in soft tissue and bone, by small blue cells with abnormal chromatin and relatively abundant pink cytoplasm, characterizing rhabdomyoblasts.[5,8]
The selection of treatment was essential for determining the prognosis.[7] In Case 1, chemotherapy and adjuvant radiotherapy were used, a common approach. For Non-Hodgkin Lymphomas, including DLBCL, the recommended chemotherapy consists of rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone.[6,7] Moreover, stem cell transplantation may be considered for recurrent or refractory conditions.[6] When discussing Case 2, which involves RMS, adjuvant or neoadjuvant chemotherapy can be used.[9] Nevertheless, surgery is typically the first step.[9] Radiotherapy is employed if the tumor cannot be fully resected.[9]
CONCLUSION
The importance of clinical suspicion, imaging tests and biopsy, when indicated, was demonstrated, even with the history and presentation indicating odontogenic orbital cellulitis. Different and varied diagnoses must be considered and evaluated immediately. All healthcare professionals, especially dentists and ophthalmologists, must be aware of diverse and rare pathologies to avoid lethal consequences or blindness.
Ethical approval:
The Institutional Review Board approval is not required.
Declaration of patient consent:
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest:
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation:
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship:
Nil.
References
- Ocular adnexal lesions: A clinical, radiological and pathological correlation Germany: Springer; 2019. p. :43-56.
- [CrossRef] [Google Scholar]
- Orbital cellulitis clinically mimicking rhabdomyosarcoma. Int Med Case Rep J. 2019;12:285-9.
- [CrossRef] [PubMed] [Google Scholar]
- Pediatric sinonasal rhabdomyosarcoma: Clinical characteristics and surgical role. Int J Surg Case Rep. 2020;2:1-6.
- [CrossRef] [Google Scholar]
- Sinonasal lymphoma: A primer for otolaryngologists. Laryngoscope Investig Otolaryngol. 2022;7:1712-4.
- [CrossRef] [PubMed] [Google Scholar]
- Orbital lymphomaan international multicenter retrospective study. Am J Ophthalmol. 2019;199:44-57.
- [CrossRef] [PubMed] [Google Scholar]
- Congenital orbital rhabdomyosarcoma. Ocul Oncol Pathol. 2017;4:165-9.
- [CrossRef] [PubMed] [Google Scholar]
- Embryonal and alveolar rhabdomyosarcoma in adults: Real-life data from a tertiary sarcoma centre. Clin Oncol. 2020;32:e27-35.
- [CrossRef] [PubMed] [Google Scholar]







