Translate this page into:
Masquerade syndrome secondary to choroidal melanoma in a 73-year-old patient

*Corresponding author: Jorge Gerardo Morales, Department of Retina and Vitreous, Ophthalmology Institute “Conde de Valenciana”, Mexico City, Mexico. jorgegerardomoralesnavarro@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Chew A, Morales JG, Fulda Graue E, Graue F. Masquerade syndrome secondary to choroidal melanoma in a 73-year-old patient. Lat Am J Ophthalmol. 2025;8:3. doi: 10.25259/LAJO_34_2024
Abstract
We present a case of masquerade syndrome secondary to choroidal melanoma, which is a rare presentation of this condition. A 73-year-old patient presented to the emergency department with a history of unilateral red, painful eye, and severe reduction in visual acuity. A Masquerade syndrome was diagnosed after slit lamp examination, and ultrasonography revealed findings suggestive of choroidal melanoma. Metastasis were ruled out, Enucleation was performed, and Histopathological results were consistent with choroidal melanoma without scleral invasion or extraocular extension. This case illustrates an unusual form of presentation for an uncommon pathology, consideration of this syndrome is of utmost importance due to a delayed diagnosis may have significant repercussions on patient mortality.
Keywords
Masquerade syndrome
Choroidal melanoma
Uveal melanoma
INTRODUCTION
Choroidal melanoma is the most common primary intraocular malignancy in adults, with an annual incidence of approximately 5.1 cases per million people in the United States.[1] Early diagnosis may prevent the risk of metastasis, thereby drastically decreasing mortality.[2] Regular ophthalmic examinations play a vital role in detecting this tumor early. Differential diagnosis includes conditions such as nevi, hemangiomas, and metastatic lesions, which can mimic the appearance of choroidal melanoma and pose diagnostic challenges. Reports suggest that skilled examiners are likely to misdiagnose its typical presentation in 1% of the cases.[3] Clinically, choroidal melanoma can present with symptoms such as blurred vision, visual field loss, or photopsia, although up to 30% of cases are asymptomatic at early stages. In cases of masquerade syndrome, where a neoplasm simulates an intraocular immune-mediated inflammatory condition, accurate diagnosis may be delayed, potentially worsening the prognosis.[4] Approximately 5% of cases initially diagnosed as uveitis are later found to be a masquerade syndrome.[5] This case report illustrates a rare presentation of choroidal melanoma as an inflammatory process in the anterior chamber, providing valuable insights into the complexities of diagnosing and managing such cases.
CASE REPORT
A 73-year-old male presented with a 1-year history of progressive visual loss with redness in his left eye, previous treatment with prednisolone acetate 1% without improvement. The patient had no significant medical history. Ophthalmologic examination showed that the best-corrected visual acuity of the right eye was 20/20, and no light perception in his left eye. Intraocular pressure was 45 mmHg in the left eye. Slit-lamp examination of the right eye showed no abnormalities. Examination of the left eye [Figure 1] revealed moderate ciliary injection, mild corneal edema, diffuse keratic precipitates, and severe anterior chamber reaction. The iris showed mild mydriasis with no reaction to light. Visualization of the posterior pole was limited by lens opacity. B-scan ultrasonography was performed [Figure 2a], revealing a homogeneous, mushroom-shaped choroidal mass measuring 5.68 mm at its largest base diameter and a thickness of 12.5 mm. A-scan ultrasonography [Figure 2b] displayed a low-medium reflectivity. Based on these findings, a masquerade syndrome secondary to choroidal melanoma was suspected. To rule out metastasis, a contrasted axial computed tomography of the thorax and abdomen was performed, showing normal results. Enucleation of the left eye was performed due to lack of vision in a painful eye. Macroscopic pathology findings [Figure 3] revealed a blackish-brown, nodular, medium-soft consistency mass of neoplastic aspect, located at the inferotemporal sector of the posterior pole of the choroid with extension to the juxtapapillary region, measuring 9 mm at its largest base diameter and a height of 11 mm. There was a total retinal detachment accompanied by abundant subretinal exudate of a yellow-greenish color. Histopathological results [Figure 4a and b] reported a malignant neoplasm composed by two cell populations, with a predominance (>60%) of tightly packed epithelioid cells with regularly defined borders, moderate amounts of cytoplasm, and round-to-oval vesicular nuclei with prominent nucleoli and few mitoses. A number of these cells displayed intracytoplasmic melanin pigment. The remaining population (<40%) consisted of compact spindle cells with poorly defined borders arranged into short bundles, with moderate amounts of cytoplasm, and ovoidto-elongated nuclei with apparent nucleoli. Similarly, some of these cells contained intracytoplasmic melanin pigment. Clusters of neoplastic cells admixed with large collections of melanophages were reported, simultaneously alternating between straight, branching, non-crosslinking, small caliber blood vessels (Folberg microvascular pattern 4). No areas of necrosis or tumor-infiltrating lymphocyte clusters were observed. The neoplasm showed neither contiguous spread nor invasion through emissaries into the sclera. Based on the results, diagnosis of mixed-cell type choroidal melanoma (epithelioid cells and spindle cells) histologic G2, tumor size category 3, without scleral invasion or extraocular extension, pathologic stage T3a N0 MO was integrated according to the American Joint Committee on Cancer (AJCC) classification of posterior uveal melanoma. This corresponds to a stage IIb.
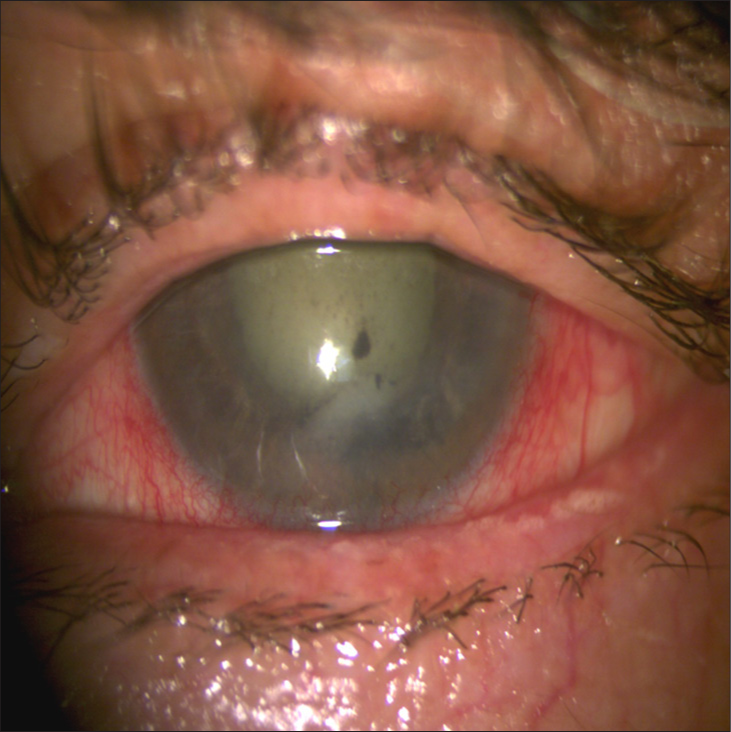
- Slit-lamp photography of the left eye showing ciliary injection, mild corneal edema, anterior chamber reaction, keratic precipitates, and nuclear sclerosis.
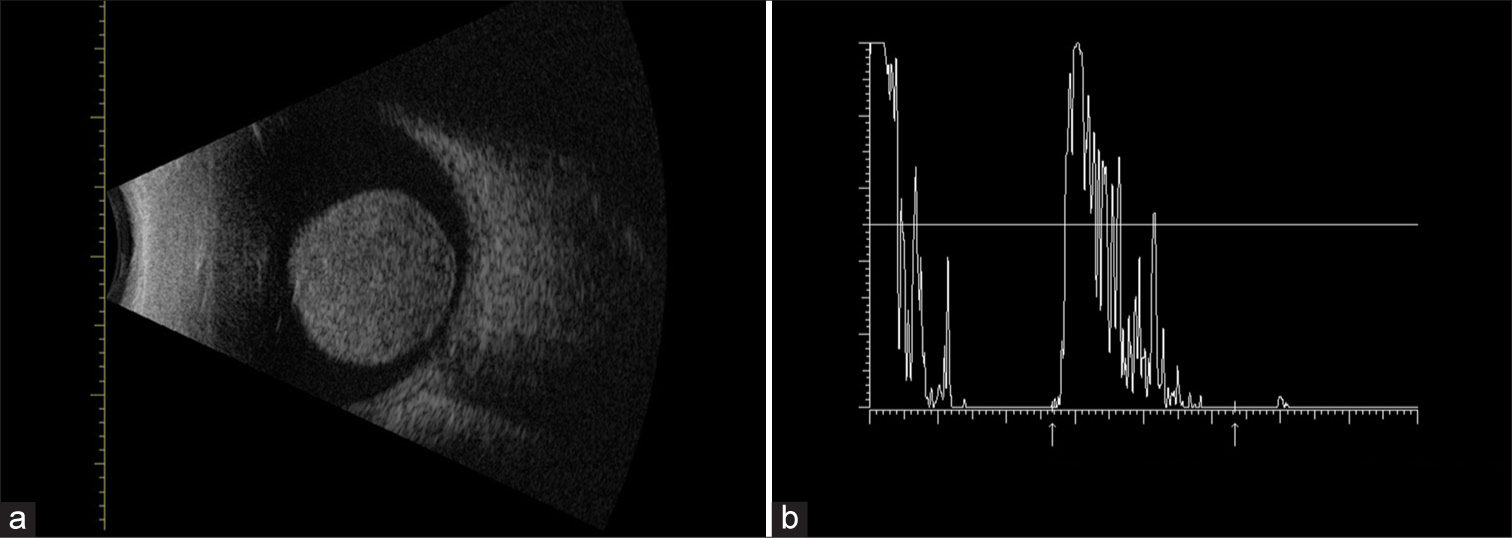
- (a) B-scan ultrasonography showing a large homogeneous mushroom-shaped mass located in the posterior pole arising from choroid. The size of the tumor measured by ultrasonography was of 12.5 mm in thickness and the largest basal dimension was 5.68 mm. Additional findings include orbital shallowing and choroidal excavation. (b) A-scan ultrasonography showing low-medium reflectivity.
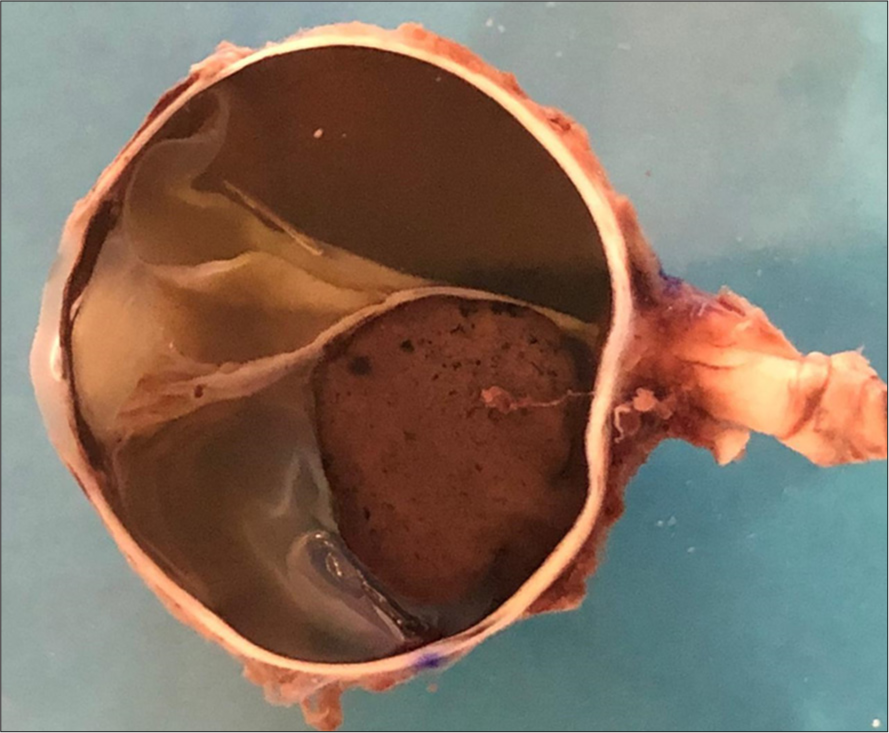
- Gross appearance of the tumor. Mass arising from the choroid at the posterior pole with extension to the juxtapapillary area, measuring 9 mm at its base and 11 mm in height. Complete detachment of the neurosensory retina, accompanied by serous subretinal fluid.
![(a) Pathologic section (Hematoxylin and Eosin [H&E], ×40) showing clusters of compact epithelioid cells admixed with melanophages. (b) Pathologic section (H&E, ×40) showing randomly disposed epithelioid cells admixed with small vessels.](/content/4/2025/8/1/img/LAJO-8-3-g004.png)
- (a) Pathologic section (Hematoxylin and Eosin [H&E], ×40) showing clusters of compact epithelioid cells admixed with melanophages. (b) Pathologic section (H&E, ×40) showing randomly disposed epithelioid cells admixed with small vessels.
DISCUSSION
Initially introduced in 1967 by Theodore when describing a conjunctival carcinoma presenting as chronic conjunctivitis, the term masquerade syndrome is typically used to refer a conditions that clinically suggest an immune-mediated uveitis without certainly being so.[6] Masquerade syndrome can occur as a manifestation of intraocular tumors, with lymphoma being the most common type.[7] Additional causes of malignant etiology include uveal melanoma, sebaceous gland carcinoma, and intraocular metastasis, while non-malignant etiologies comprise ocular ischemic syndrome, chronic retinal detachment, and retinitis pigmentosa.[8] In patients over the age of 60, uveitis can present as a masquerade syndrome in up to 1.5–5.3% of the cases, 95% of which occur in Caucasian patients aged 50 years, with unilateral presentation and posterior pole involvement.[9,10]
Choroidal melanoma accounts for 54% of all uveal melanomas[11] and presents asymptomatically in up to 30% of cases.[12,13] Rarely, an anterior chamber reaction is the sole finding upon ophthalmologic examination. In such cases, the reaction observed is caused by tumoral, pigmentary, and inflammatory cells, as opposed to cases of immune-mediated uveitis, in which T-cells are predominant.[14] There have been reports suggesting a release of cytokines and inflammatory mediators from necrotic tissue in advanced melanomas, manifesting as anterior uveitis, panuveitis, or episcleritis.[15] Moreover, studies have found that up to 4.9% of uveal melanomas may present with signs of inflammatory reaction, such as anterior or posterior uveitis, episcleritis, and endophthalmitis.[15] Case reports published on the subject addressed by this article are scarce throughout the literature and include the work published by Macedo and collaborators, in which a definitive diagnosis was made upon performance of a biopsy for liver metastasis in a patient initially classified with chronic endophthalmitis.[16] Other reported cases describe onset presentation in the form of chronic uveitis with no response to steroid treatment, spontaneous anterior chamber hemorrhage, and choroidal abscess secondary to endogenous endophthalmitis with associated recurrent hypopyon.[15,17,18]
Diagnostic tools such as slit-lamp examination, B-scan ultrasonography, and A-scan ultrasonography are crucial in identifying the underlying neoplastic nature of such conditions. However, these tools have limitations, particularly in cases with media opacity or when the tumor’s characteristics mimic inflammatory processes.[13]
Definitive diagnosis is made by fine-needle aspiration biopsy. Once the diagnosis is established, further systemic investigations should be conducted to rule out metastatic disease.[16] While only <4% of patients present with detectable metastasis upon diagnosis, nearly half of them are expected to develop metastasis at a later stage. The most common sites of systemic metastasis are the liver, lungs, skin, and connective tissue.[19] It is estimated that the median overall survival rate after the progression of metastatic disease is of approximately 13.4 months.[19] The management of choroidal melanoma requires a multidisciplinary approach involving ophthalmologists, oncologists, radiologists, and pathologists to ensure accurate diagnosis and comprehensive care. Treatment strategies vary based on tumor size, location, and progression and may include observation, photocoagulation, transpupillary thermotherapy, radiotherapy, and surgical options such as enucleation or exenteration.[13]
Differential diagnoses
Clinically, a masquerade syndrome may “mask” or mimic a neoplastic etiology.[13] Despite it being an unusual form of presentation for an uncommon pathology, consideration of this syndrome is of utmost importance. In cases of uveitis that does not respond to initial treatment, late diagnosis could lead to increased mortality and morbidity. Due to the complexity involved in establishing the diagnosis, ultrasonography is useful in cases of media opacity as well as in the assessment of the peripheral uveal tract. Findings supporting the diagnosis include a low-medium internal reflectivity, choroidal excavation, and orbital attenuation.[8]
Early diagnosis of choroidal melanoma can have an impact on both treatment plan and survival.[20] Tumor size at the time of enucleation, as an indicator of risk of metastasis and, thus of survival, has been found to be the primary prognostic factor.[21] A review conducted by Diener-West on the 5-year mortality from choroidal melanoma reported a significant impact on survival based on the size of the tumor, with mortality rates varying from 16% for small tumors (<3 mm in height and <10 mm in diameter), 32% for medium tumors (<15 mm in diameter), and 53% for large tumors (>15 mm in diameter or >5 mm in height).[18] These statistics highlight the importance of early detection and intervention.
Likewise, Arnljotsk et al. found that 10-year relative survival rates for small, medium, and large tumors ranged from 62%, 44%, and 31%, respectively, as compared to survival rates when considering the categories defined by the AJCC in its 8th Edition Staging Manual, being that of 50%, 45%, 56%, and 0% for T1, T2, T3, and T4, respectively.[21] In addition, evidence showed that 5-year mortality rate without undergoing treatment reached 30% (confidence interval [CI], 18–47%), as opposed to a mortality rate of 18% (CI, 16–20%) when receiving protocol treatment (radiotherapy and/or enucleation).[22]
Treatment modalities include observation, photocoagulation, transpupillary thermotherapy, radiotherapy (external bean radiation/brachytherapy), local resection, enucleation, and exenteration. The approach to be chosen depends on visual acuity, intraocular pressure, size, growth pattern, and location of the tumor.[23] Further discussion on the management of this pathology is beyond the scope of this paper.
CONCLUSION
Ninety-five percent of choroidal melanoma cases can be correctly diagnosed through indirect ophthalmoscopy as well as multimodal imaging and ultrasonography. However, diagnosis can become challenging when the presentation of the disease is atypical. Therefore, in cases of uveitis with a slow progression and inadequate response to conventional treatment, the possibility of a masquerade syndrome and its underlying causes should be considered. A delayed diagnosis may have significant repercussions on the patient mortality.
Ethical approval
The Institutional Review Board has waived the ethical approval for this study.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There is no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Choroidal melanoma: Natural history and management options. Cancer Control. 2004;11:296-303.
- [CrossRef] [PubMed] [Google Scholar]
- Very long-term prognosis of patients with malignant uveal melanoma. Invest Ophthalmol Vis Sci. 2003;44:4651.
- [CrossRef] [PubMed] [Google Scholar]
- Accuracy of diagnosis of choroidal melanomas in the collaborative ocular melanoma study. Arch Ophthalmol. 1990;108:1268.
- [CrossRef] [PubMed] [Google Scholar]
- Conjunctival carcinoma masquerading as chronic conjunctivitis. Eye Ear Nose Throat Mon. 1967;46:1419-20.
- [Google Scholar]
- Neoplastic masquerade syndromes. Surv Ophthalmol. 2002;47:81-124.
- [CrossRef] [PubMed] [Google Scholar]
- Neoplastic masquerade syndromes in patients with uveitis. Am J Ophthalmol. 2014;157:526-31.
- [CrossRef] [PubMed] [Google Scholar]
- Incidence of uveal melanoma in the United States: 1973-1997. Ophthalmology. 2003;110:956-61.
- [CrossRef] [PubMed] [Google Scholar]
- Uveal melanoma: Epidemiology, etiology, and treatment of primary disease. Clin Ophthalmol. 2017;11:279-89.
- [CrossRef] [PubMed] [Google Scholar]
- Uveitis caused by cytotoxic immune response to cutaneous malignant melanoma in swine: Destruction of uveal melanocytes during tumor regression. Invest Ophthalmol Vis Sci. 1983;24:1063-9.
- [Google Scholar]
- Necrotic choroidal melanoma masquerading as scleritis. Indian J Ophthalmol. 2020;68:1979.
- [CrossRef] [PubMed] [Google Scholar]
- Choroidal melanoma masquerading as panuveitis in a patient with multiple myeloma. Retin Cases Brief Rep. 2009;3:152-5.
- [CrossRef] [PubMed] [Google Scholar]
- Ocular melanoma presenting as masquerade syndrome. Eur J Case Rep Intern Med. 2019;6:001118.
- [Google Scholar]
- Ocular inflammation and hemorrhage as initial manifestations of uveal malignant melanoma. Arch Ophthalmol. 1979;97:1311.
- [CrossRef] [PubMed] [Google Scholar]
- Uveal melanoma in the peripheral choroid masquerading as chronic uveitis. Optom Vis Sci. 2014;91:e222-5.
- [CrossRef] [PubMed] [Google Scholar]
- A review of mortality from choroidal melanoma. Arch Ophthalmol. 1992;110:245.
- [CrossRef] [PubMed] [Google Scholar]
- Prognosis of mucosal, uveal, acral, nonacral cutaneous, and unknown primary melanoma from the time of first metastasis. Oncologist. 2016;21:848-54.
- [CrossRef] [PubMed] [Google Scholar]
- Quality of life after iodine 125 brachytherapy vs enucleation for choroidal melanoma: 5-year results from the Collaborative Ocular Melanoma Study: COMS QOLS Report No. 3. Arch Ophthalmol. 2006;124:226-38.
- [CrossRef] [PubMed] [Google Scholar]
- Tumour thickness, diameter, area or volume? The prognostic significance of conventional versus digital image analysis-based size estimation methods in uveal melanoma. Acta Ophthalmol. 2018;96:510-8.
- [CrossRef] [PubMed] [Google Scholar]
- Mortality after deferral of treatment or no treatment for choroidal melanoma. Indian J Ophthalmol. 2018;66:1395-400.
- [CrossRef] [PubMed] [Google Scholar]
- Uveal melanoma treatment and prognostication. Asia Pac J Ophthalmol. 2017;6:186-96.
- [Google Scholar]







