Translate this page into:
Through the looking glass: Ciliary body leiomyoma with chronic inflammation: A rare case report

*Corresponding author: Twinkey Bhutia, Department of Vitreo-Retina, Sri Sankaradeva Nethralaya, Guwahati, Assam, India. twinkey.bhutia@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Bhutia T, Das D, Barman M. Through the looking glass: Ciliary body leiomyoma with chronic inflammation: A rare case report. Lat Am J Ophthalmol. 2024;7:11. doi: 10.25259/LAJO_14_2024
Abstract
Ciliary body leiomyoma is an exceptionally rare tumor arising from the smooth muscle cells of the ciliary body. We present a unique case of a patient who came with a diminution of vision, pain, and redness in the left eye post blunt trauma, which was diagnosed as scleritis elsewhere. Ophthalmic examination revealed panuveitis, complicated cataract, and tractional retinal detachment. During surgery, a ciliary body mass was encountered, and the left eyeball was subsequently enucleated. Histopathological examination confirmed the diagnosis of ciliary body leiomyoma with chronic inflammation. This case highlights the rarity and complexity of ciliary body leiomyoma which often presents as a diagnostic challenge and, therefore, deserves in-depth exploration and documentation in scientific literature.
Keywords
Ciliary body
Leiomyoma
Panuveitis
Chronic inflammation
Diagnostic dilemma
Case report
INTRODUCTION
Leiomyomas are exceedingly rare benign smooth muscle tumors, predominantly found in the female reproductive organ, the uterus,[1] and the alimentary tract. However, isolated cases of uveal leiomyomas, affecting the iris, ciliary body, and choroid have been documented.[2] These benign tumors exhibit locally destructive behavior and cause both intraocular and extraocular complications. Ciliary body leiomyomas can be of myogenic or neurogenic[3] origin. The precise pathophysiology and etiology of leiomyomas remain unclear, but emerging evidence points to the role of chronic inflammation in promoting their growth.
CASE REPORT
A 49-year-old male presented with a diminution of vision and redness in the left eye (OS) for 1 month, which was painful, gradual, and progressive in nature. There was a history of blunt trauma with a bamboo stick 4 years back, for which he was diagnosed with diffuse scleritis elsewhere. His best-corrected visual acuity in the right eye (OD) was 6/6, N/6, and OS was perception of light, and inaccurate projection of rays. External examination of the eye was within normal limits. Slit-lamp examination in OD showed normal anterior segment while OS showed circumciliary congestion, anterior chamber cells 4+, flare 3+ with 360° posterior synechiae. Lens had nuclear sclerosis Grade 2. The intraocular pressure of both the eyes (OU) was 12 mmHg.
Fundus examination was normal in OD and OS could not be examined due to media haze. Ultrasound B-scan of OS showed low-to-moderate reflective membrane echoes in the vitreous intragel with focal traction and shallow retinal detachment. The patient was diagnosed with panuveitis, complicated cataracts, and tractional retinal detachment in the OS. Blood investigations,such as complete blood count and erythrocyte sedimentation rate were normal, while human immunodeficiency virus, Venereal disease research laboratory, and Mantoux tests were negative. The patient was planned for retinal detachment surgery in the OS under oral steroid cover, but during surgery, severe vitreous hemorrhage with a ciliary body mass was detected. Immediate vitreous aspiration and cytology revealed atypical cells. Magnetic resonance imaging brain and orbit showed a 12 × 6 mm plaque-like mass in the temporal aspect of OS, which was hyperintense on the T1-weighted (T1W) image [Figure 1a] and hypointense in the T2-weighted (T2W) image. No extraocular extension was noted. A provisional diagnosis of melanoma, metastasis, or hemangioma was made. Subsequently, OS was enucleated with proper consent. In gross examination, the optic nerve measured 3.76 mm in length and 3.31 mm in thickness. Transillumination defect was not seen. A light, brown-colored ciliary body mass was noted with exudative retinal detachment (ERD) with suspected scleral involvement [Figure 1b] and chronic inflammation [Figure 1c]. Microscopically, there was episcleritis and scleritis [Figure 2a], trabeculitis [Figure 2b], focal iris neovascularization, and hemorrhage. The mass consisted of well-differentiated paucicellular spindle cells [Figure 2c], epithelioid cells, fibrous tissues, and lymphocytic infiltration with no mitosis. The adjoining optic nerve was normal and on immunohistochemistry (IHC), the tumor stained positive for Vimentin, S 100, CD 3, and CD20 [Figure 3]. It stained negatively for melanoma-specific markers (HMB-45). Hence, based on the microscopic appearance of the tumor and its IHC characteristics, a final diagnosis of OS ciliary body leiomyoma with chronic inflammation was made.
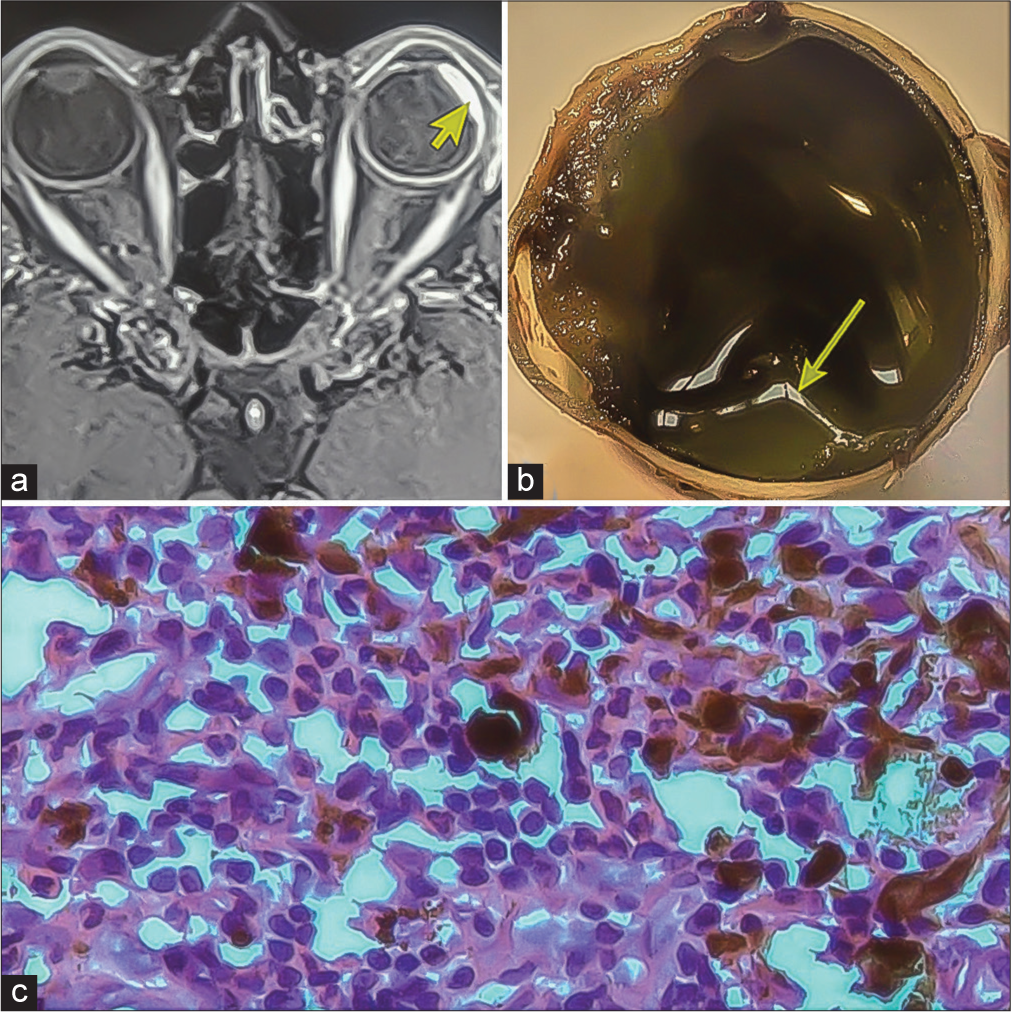
- (a) Magnetic resonance imaging showing 12 × 6 mm hyperintense plaque-like mass (yellow arrowhead) in T1-weighted image. (b) Light-pigmented mass near the ciliary body (yellow arrow). (c) Microscopic examination showing chronic inflammation.
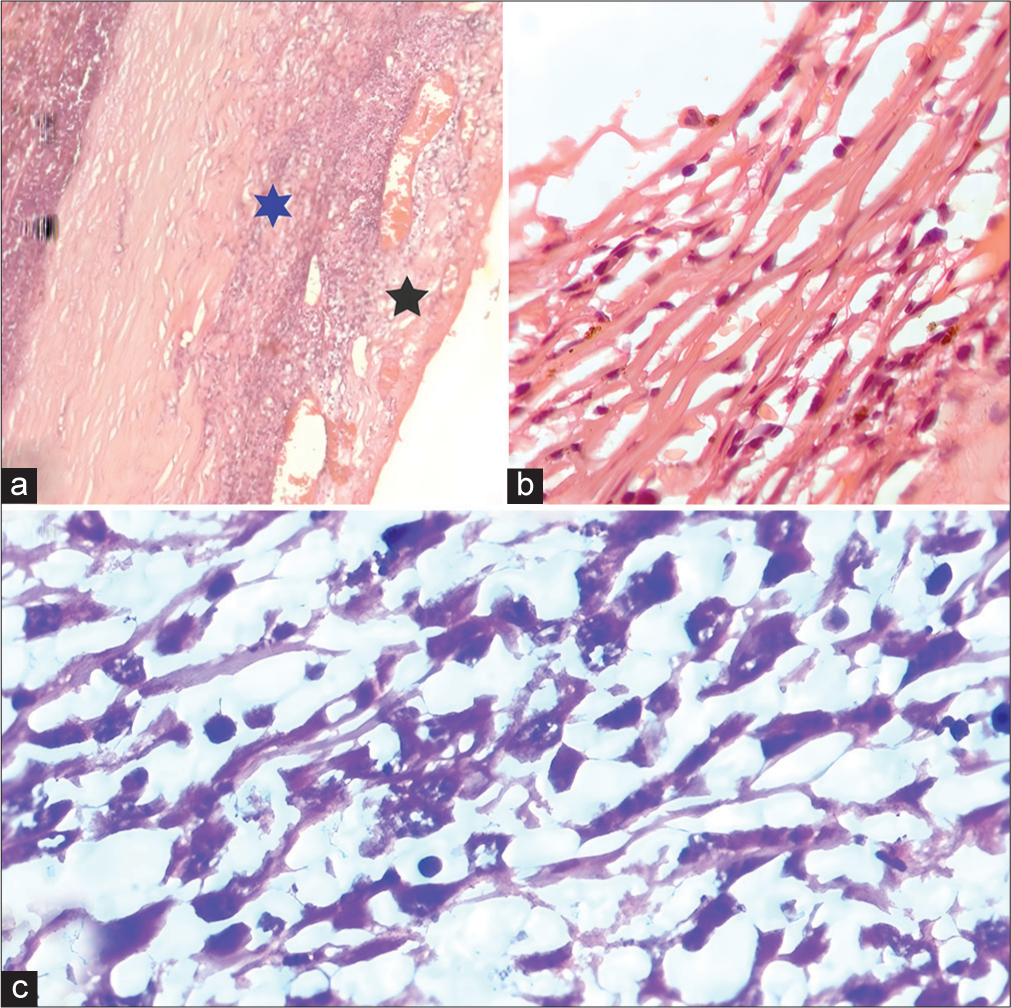
- (a) Microscopic examination (ME) showing episcleritis (black star) and scleritis (blue star). (b) ME showing trabeculitis. (c) Showing well-differentiated paucicellular spindle cells.
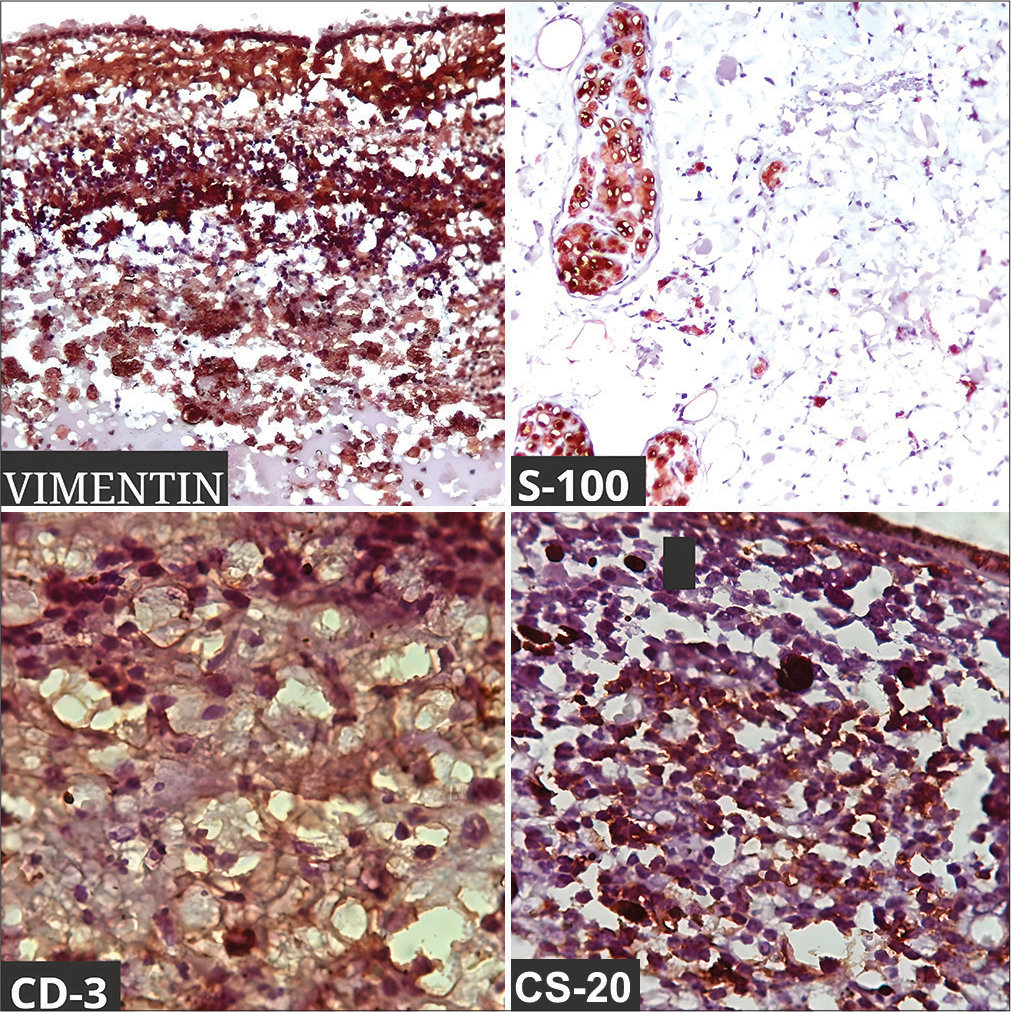
- Immunohistochemical stain positive with Vimentin, S-100, CD-3, and CD-20.
DISCUSSION
Leiomyomas are rare benign smooth muscle tumors arising from the neural crest cells. A post-enucleation ciliary body leiomyoma was discovered by Fuchs and coworkers as early as 1928 and was subsequently verified by Blodi in 1950. Usually, it has a female preponderance affecting the 3rd and 4th decades of life. However, in our case, the patient affected was a male. Tomar et al.[4] in their case series also had two patients who were of the male gender. They most commonly present with symptoms of progressive diminution of vision. Rarely, they can present with unusual symptoms such as recurrent episcleritis or scleritis as in our case. Some patients who presented with anterior staphyloma have also been reported in the literature.[5,6] Although the origin and pathophysiology of leiomyomas are unclear, the role of chronic inflammation has been explored as a potential factor for tumor growth. The specific cytokines secreted by the immune, undifferentiated, and tumor cells sustain the chronic inflammation[7] and influence proliferation, fibrosis, and angiogenesis, thereby promoting the growth of leiomyomas. The progenitor cells play a central role in the microenvironment and modulate the cellular functions of immune cells, including B and T lymphocytes, natural killer cells, monocytes, and dendritic cells. Presumably, this role is operated by a complex interplay of short- and long-range signaling that may entail a wide spectrum of molecular mediators, including soluble cytokines and growth factors. Although classified as benign and slow-growing, ciliary body leiomyomas are locally destructive and can cause scleral ectasia, extrascleral extensions, cataracts, secondary ERDs, and neovascular glaucoma. Leiomyomas are smooth, ovoid-shaped, and amelanotic rubbery masses that are sometimes covered by a pigmented layer. They are composed of intracellular myoglial fibrils, interlacing spindle cells with oval nuclei, and eosinophilic fibrillary cytoplasm. Due to their organized cellular arrangements, nuclear palisading, and lack of melanin, leiomyomas are translucent to transillumination. However, in our case, the transillumination was absent. Tomar et al., in their review, noted that out of 80 patients, only 29 of them had transillumination.[4] The differential diagnosis can be amelanotic uveal melanoma, ciliary body metastases, leiomyosarcoma, neurofibroma, and schwannoma. Distinguishing between uveal melanoma and ciliary body leiomyoma presents a diagnostic challenge as they share similar light microscopic and radiological characteristics. They both appear hypointense in T2W images and hyperintense in T1W images. Fine-needle aspiration biopsy can be done to aid in the diagnosis. Leiomyomas are uniformly reactive to α-smooth muscle actin, muscle-specific actin,[8] desmin, vimentin, and h-caldesmon. They stain positive with S-100, neuron-specific enolase, and CD-56 due to their neuroectodermal origin, and stains negative for melanoma-specific markers (HMB-45 and Melan-A). In our case report, the tumor stained positive for Vimentin, S-100, CD3, and CD20 and was negative for the melanoma-specific marker (HMB-45). Quhill et al. have made an interesting analysis where they found the tumor cells stained positive to two sex steroid hormone receptors (progesterone and androgen but not estrogen receptors).[9] Alkatan et al. also have reported similar reactivity to progesterone but not estrogen receptors.[10] Immunohistochemical staining, along with histopathology, therefore, serves as the gold standard for diagnosing ciliary body leiomyomas.
The management of leiomyoma depends on tumor size, location, and secondary ocular complications. Observation of small ciliary body leiomyomas is rarely pursued due to the challenge of excluding amelanotic melanoma in most cases. Tumors involving 3–4 clock hours can be removed by transscleral resection through partial lamellar sclerouvectomy or endoresection through a pars plana vitrectomy but it can cause vitreous hemorrhage, retinal, and hemorrhagic choroidal detachment. Radiation therapy can be pursued for small growing tumors, but it has side effects such as skin excoriation, cataract formation, radiation retinopathy, and neovascular glaucoma. Treatment needs to be tailored on a case-to-case basis; however, enucleation remains the definitive treatment approach in advanced stages as in our case, for the management of uveal leiomyomas.
CONCLUSION
Ciliary body leiomyoma with chronic inflammation is a very rare clinical entity that often gets misdiagnosed with other tumors and inflammatory conditions. A thorough and proper examination, including histopathology and IHC is necessary to diagnose the tumor early and prevent late complications. Further, research to investigate the response of tumors to hormonal therapy and other treatment modalities can be explored in the future.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Leiomyoma of the ciliary body. Am J Ophthalmol. 1950;33:939-42.
- [CrossRef] [PubMed] [Google Scholar]
- Mesectodermal leiomyoma of the ciliary body. A tumor of presumed neural crest origin. Cancer. 1977;39:2102-13.
- [CrossRef] [PubMed] [Google Scholar]
- Intraocular leiomyoma: Current concepts. Surv Ophthalmol. 2020;65:421-437.
- [CrossRef] [PubMed] [Google Scholar]
- Observations on seven cases of intraocular leiomyoma. The 1993 byron demorest lecture. Arch Ophthalmol. 1994;112:521-8.
- [CrossRef] [PubMed] [Google Scholar]
- Mesectodermal leiomyoma of the ciliary body presenting as anterior staphyloma. Retin Cases Brief Rep. 2017;11:266-8.
- [CrossRef] [PubMed] [Google Scholar]
- Inflammation-induced cancer: Crosstalk between tumours, immune cells and microorganisms. Nat Rev Cancer. 2013;13:759-71.
- [CrossRef] [PubMed] [Google Scholar]
- A leiomyoma of the iris documented by immunohistochemistry and electron microscopy. Acta Ophthalmol Scand. 1997;75:470-3.
- [CrossRef] [PubMed] [Google Scholar]
- Three cases of intraocular mesectodermal leiomyoma expressing progesterone and androgen receptors. Eye (Lond). 2013;27:669-72.
- [CrossRef] [PubMed] [Google Scholar]
- A case of ciliary body mesectodermal leiomyoma with rapid growth and loss of vision necessitating enucleation. Ann Med Surg (Lond). 2020;60:651-3.
- [CrossRef] [PubMed] [Google Scholar]






