Translate this page into:
Pachychoroid as a biomarker using optical coherence tomography – swept-source in central serous choroidopathy

*Corresponding author: Tatiana Urrea-Victoria, Institute of Ophthalmology Foundation Conde de Valenciana, National Autonomous University of Mexico, Chimalpopoca 14, Colonia Obrera, Mexico City, Mexico. tatianaurre@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Cano-Hidalgo RA, Urrea-Victoria T. Pachychoroid as a biomarker using optical coherence tomography – swept-source in central serous choroidopathy. Lat Am J Ophthalmol 2019;2(3):1-5.
Abstract
Objective
The objective of the study was to describe and evaluate the subfoveal choroidal thickness (SFCT) in the fellow eyes of patients with central serous chorioretinopathy (CSC) using swept source optical coherence tomography (SS-OCT).
Methods
This was a transversal, retrospective, and observational study. The SFCT was measured in patients with unilateral CSC using SS-OCT. The choroidal thickness in symptomatic and fellow eyes was measured using the attached measuring software in SS-OCT. The SFCT dimension was obtained from the horizontal section under the foveal center from the OCT data and these data were analyzed.
Results
The mean age of subjects undergoing imaging SS-OCT was 44.23 years old (standard deviation, 11.57). 30 out of 60 patients (63.3%) were men, and 20 (33.3%) patients had acute clinical disease. The median choroidal thicknesses of the affected eyes were greater than those of the unaffected fellow eyes (P = 0.06). The choroidal thickness measured in 120 eyes of (60 patients) was 421 µm (interquartile range 352–490), which was greater than the choroidal thickness reported in normal eyes.
Conclusions
The measuring of the choroidal thickness using SS-OCT is useful as a non-invasive technique to evaluate the subclinical choroidal abnormalities in CSC.
Keywords
Central serous chorioretinopathy
Choroidal thickness
Optical coherence tomography
Pachychoroid
Swept-source optical coherence tomography
INTRODUCTION
The term pachychoroid (pachy-[prefix]: Thick) is defined as a permanent and abnormal increase in the choroidal thickness, due to dilatation of the large outer choroidal vessels (Haller’s layer), compressing the overlying choriocapillaris and Sattler’s layer.[1] This disease spectrum refers to a group of retinochoroidal disorders that share distinctive choroidal findings identified with multimodal retinal imaging.[2] Recent progress in retinal imaging based on enhanced depth imaging- optical coherence tomography (EDI-OCT) and swept-source OCT (SS-OCT) technologies, has provided new insights and enabled a precise structural and functional analysis of the choroid.[3] One of the diseases of the spectrum is central serous chorioretinopathy (CSC), a condition characterized by exudative detachment of the neurosensory retina, usually seen in young men.[4]
The pathophysiology of CSC is not completely understood, although it was previously that the retinal pigment epithelium (RPE) was involved in its genesis, in 1967 Gass, proposed that choriocapillary hyperpermeability may be the cause.[5,6] As the primary pathology in the CSC is in the choroid, studies with EDI-OCT have shown that patients with unilateral involvement also have increased choroidal thickness in the unaffected eye,[4] which suggests that there may be bilateral elevated hydrostatic pressure in the choroidal vasculature in CSC.[4]
CSC studies using indocyanine green angiography (ICGA) have revealed that the hyperpermeability of choroidal vessels is much higher than expected compared with the RPE leak observed in fluorescein angiography. It is believed that this increased permeability of the choroidal vasculature and the dysfunction of the RPE barrier play an important role in the pathophysiology of the disease.[4] The hyperpermeability might cause the choroidal thickening through the accumulation of fluid, and the expansion of the choroidal vessels could play a partial role for the choroidal thickening.[5]
It has been reported that the choroidal thickness based on histological samples varies from 170 to 220 µm, being thinner than that reported in recent measurements in vivo by spectral domain OCT (SD-OCT).[7,8] Normal choroidal thickness was first described by Margolis and Spaide using the SD-OCT (Spectralis Heidelberg Engineering Germany) and Manjunath and collaborators using the Cirrus HD-OCT (Carl Zeiss Meditec, Dublin, Ca, Usa), as a result, they found that the choroid was thicker under the fovea and thinner in the nasal retina compared to the temporal. The subfoveal choroidal thickness (SFCT) reported was 287 ± 76 μm with spectralis and 272 ± 81 µm with cirrus.[4]
It is still unclear if there is an increase in bilateral choroidal thickness in patients with unilateral CSC, since in some studies with ICGA, only up to 62% of bilateral choroidal vascular hypermeability has been reported, which indicates that the involvement of both eyes is not always expected.[9]
Maruko et al. reported that although the choroid was thicker in the fellow eyes with choroidal vascular hyperpermeability on ICGA, the choroid was not thicker in the fellow eyes without hyperpermeability.[5] The measuring of choroidal thickness using SS-OCT is useful to noninvasively evaluate the subclinical choroidal abnormalities in CSC.[5] The purpose of this article is to describe the SS-OCT findings seen in CSC disease.
METHODS
Ethics
The study was approved and was conducted in accordance with the ethical standards stated in the 1964 Declaration of Helsinki.
Study design
This was a retrospective, observational, and transversal study.
Methods
Patients medical records were reviewed, including best-corrected visual acuity, evaluation with a dilated pupil of fundus photography, and imaging data SS-OCT. SFCT was manually measuring using the caliper tool software of the SS-OCT. SFCT was defined as the distance from the outer portion of the hyper-reflective line (corresponding to the RPE) to the inner surface of the choroidal-scleral junction. Images were set to an aspect ratio of 1:1 mm before each measurement. If the choroidal scleral junction was not clearly identified, brightness and contrast of the image were adjusted, using the built-in adjustment tool, to best visualize this junction, and the outer choroidal border was identified by the line connecting the outer margin of the large choroidal vessel layer. The choroidal thickness was measured in the location corresponding to the focus of subfoveal subretinal fluid [Figures 1 and 2]. Patients were excluded if they had any history of inflammatory eye disease or if they had drusen.
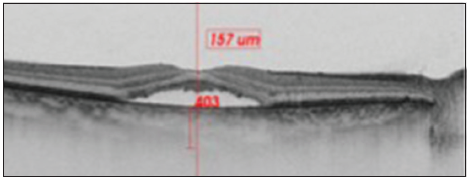
- The choroid is seen in cross-section using swept–source- optical coherence tomography. Subfoveal choroidal thickness was measured vertically from the outer border of the retinal pigment epithelium to the inner border of the sclera.

- The choroid is seen in cross-section using swept source- optical coherence tomography. Subfoveal choroidal thickness was measured vertically from the outer border of the retinal pigment epithelium to the inner border of the sclera.
Statistical analysis
Numerical data were summarized using means, standard deviations, medians, and interquartile ranges (IQRs), while categorical data were summarized using frequencies and percentages. The results of the measurement of the choroidal thickness were analyzed using the nonparametric Kruskal–Wallis test and Chi-square test was used to test differences between proportions. P < 0.05 was considered statistically significant. Statistical analyses were performed using SPSS software version 25.0 (Ibm, Armonk, Ny, USA).
RESULTS
A total of 120 eyes from 60 patients with a mean age of 44.23 ± 11.57 years old presenting CSC were analyzed. The patients with CSC were 63.3% men aged 43.37 ± 10.74 years old. The choroidal thickness measured was 421 µm (IQR 352–490); median choroidal thickness was 435 µm, (IQR, 380–524 µm) in affected eye with CSC and 397µm (IQR, 334–461 µm) in the fellow eye.
The cutoff point of choroidal thickness was established in the 75th percentile of our sample obtaining 490 µm. 15 eyes (25%) showed choroidal thickness >490 µm. Negative low correlation was found between choroidal thickness value and age, (spearman’s rho correlation coefficient – 0.34; P = 0.008) [Figures 3 and 4]. The median difference in choroidal thickness between the affected eye and the fellow eye was 41 µm (P = 0.06). 28 (46.7%) of the eyes with CSC had visual acuity equal or better than 20/40. Fundus examination showed serous detachment foveal and extrafoveal in all of them, 53 (44.16%) with retinal pigment epithelial changes. No hemorrhages or choroidal neovascularization were observed. There was no correlation between choroidal thickness and visual acuity in the eye with CSC and retinal detachment (RD) (Spearman’s Rho correlation coefficient 0.019; P = 0.90) [Figure 5]. There was no significant difference between the mean SFCT and sex [Figure 6]. The median choroidal thickness was significantly (P < 0.05) greater in the affected eye with CSC compared with the fellow eye in patients with age 40–49 years old; however, no significant differences were detected in the other ages. The SFCT in male eyes with CSC were significantly (P < 0.05) greater than that in the fellow eyes. Demographic and clinical data are summarized in Tables 1 and 2.
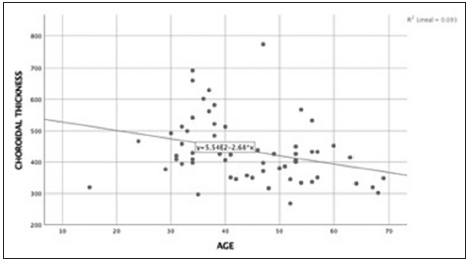
- Correlation age and choroidal thickness. Spearman’s coefficient correlation was – 0.34 (P = 0.008). There was a negative low correlation between age and choroidal thickness.
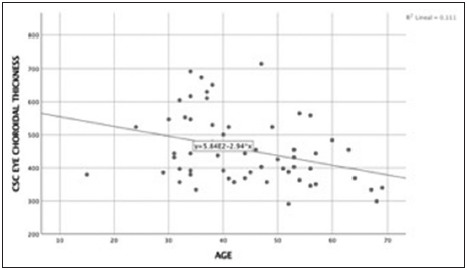
- Correlation age and central serous chorioretinopathy eye choroidal thickness. Spearman’s coefficient correlation was – 0.33 (P = 0.009). There was a negative low correlation between age and choroidal thickness.
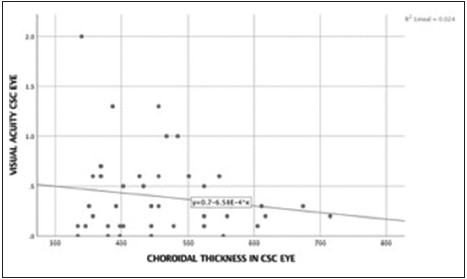
- Correlation best corrected visual acuity and choroidal thickness in central serous chorioretinopathy eye. Spearman’s coefficient correlation was 0.019 (P = 0.90). There was no correlation between best corrected visual acuity in central serous chorioretinopathy eye and choroidal thickness in central serous chorioretinopathy eye.
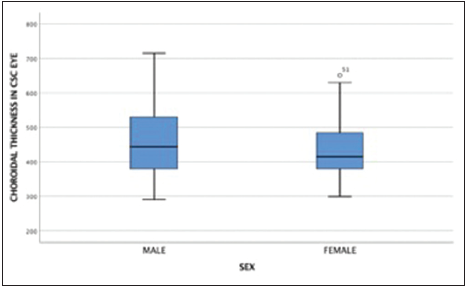
- Box plot distribution of choroidal thickness measurements in central serous chorioretinopathy eye by sex.
| Clinical characteristics | Choroidal thickness | |||
|---|---|---|---|---|
| <490 μm (%) | >490 μm (%) | |||
| Age (years) | ||||
| <40 | 14 (31.1) | 12 (80)* | ||
| >40 | 31 (68.9) | 3 (20)* | ||
| Sex | ||||
| Male | 27 (60) | 11 (63.3) | ||
| Female | 18 (40) | 4 (26.7) | ||
| Stage | ||||
| Acute | 12 (30) | 8 (53.3) | ||
| Chronic | 28 (70) | 7 (46.7) | ||
| Visual acuity | ||||
| <20/40 | 19 (57.6) | 9 (81.8) | ||
| >20/50 | 14 (47.4) | 2 (18.2) | ||
Chi-square test *P<0.05
| Clinical characteristics | CSC eye choroidal thickness (μm) | Fellow eye choroidal thickness (μm) | ||
|---|---|---|---|---|
| Choroidal thickness (μm) | 435 (380–524) | 397 (334–456)* | ||
| Age<39 years old | 501 (394.5–608) | 432 (388.5–513) | ||
| Age 40–49 years old | 403 (369–501) | 345 (328–432)* | ||
| Age>50 years old | 398 (351–455) | 374 (305–420) | ||
| Sex | ||||
| Male | 444 (380–530) | 403.5 (345–478)* | ||
| Female | 415 (380–484) | 388.5 (305–432) | ||
| Foveal involvement (RD) | 414.5 (384.5–479.5) | 391.50 (334–444) | ||
| Extrafoveal involvement (RD) | 449.5 (367–550) | 397 (332–513) | ||
Median and IQR. *Kruskal–Wallis test P<0.05. RD: Retinal detachment, CSC: Central serous chorioretinopathy, IQR: Interquartile range
DISCUSSION
The quantitative evaluation of the choroid has been challenging the traditional imaging modalities, such as ICGA and ultrasonography, due to the limited resolution and repeatability. With the advent of OCT, detailed visualization of the choroid is possible in vivo, and the role as a non-invasive way of studying the retina is well known.[10] The measurements of the choroidal thickness have also allowed new directions in the investigation of the study of normal and pathological processes within the choroid.[4]
One of the drawbacks of EDI-OCT is that in certain eyes, especially in those with media opacities, relatively clear images of the retina can be obtained, but the junction of the choroid and sclera cannot be clearly visualized, which is possible using the SS-OCT. Point-to-point measurements of the choroid, however, are a problematic method not only due to operator bias but also due to the highly variable choroidal anatomic features. Unlike the retina, the choroid is a highly vascularized structure with significant diurnal fluctuations in thickness.[11] Although measuring the thickness of the choroid may be more challenging than measuring retinal thickness, due to high local variability of the choroid; using SS-OCT is useful as a very reliable and non-invasive technique to evaluate the subclinical choroidal abnormalities.[11]
Based on the above, it was decided to perform a study of choroidal thickness measurement in patients with CSC using SS-OCT as it allows in vivo visualization of the choroid, appropriate measurement and since it is a non-invasive reproducible technology with high-resolution it allows the measurement and identification of choroidal findings in our population.
It has been suggested that the increase in the frequency of bilateral cases is higher in long-term follow-up studies; therefore, unilateral cases of CSC with bilateral choroidal vascular hyperpermeability could become bilateral cases during follow-up.[5] The frequency in the report of bilateral cases with CSC varies from 4% to 14% in cross-sectional studies and from 20% to 30% in longitudinal observational studies.[5] A follow-up is necessary to determine possible variations in choroidal thickness and to establish the prevalence of bilateral affectation by longitudinal studies.
Imamura et al. reported an increase in bilateral choroidal thickness in patients with CSC, even in eyes without RD,[5] data that correlate with the one found in this current study. As reported in literature, in our study the choroid thickness in patients with CSC did not show a correlation with age and did not demonstrate the age-related decline seen in patients with normal eyes.[12]
The choroid was thick in both eyes, even if the subretinal fluid was seen in only one eye. Pachychoroid may support the theory of the change of the choroidal vessels and might be used as a biomarker to predict the development of CSC in the fellow eye.
The follow-up and understanding of tomographic findings of patients with CSC allow the implementation of the monitoring of the disease in the future.
CONCLUSIONS
The study revealed that the subfoveal choroid in the fellow eye of patients with CSC was thicker than normal.
The SS-OCT, a reproducible and non-invasive technology, allows in vivo visualization of the choroid and the appropriate measurement with high resolution.
The measuring of the choroidal thickness using SS-OCT is useful to evaluate the subclinical choroidal abnormalities in CSC.
The current study had weaknesses including the fact that it was retrospective, with small sample size.
Increased choroidal thickness in the fellow eye could play a predictive factor in the development of bilateral CSC so; it is necessary to perform longitudinal studies.
Declaration of patient consent
A retrospective study was carried out. This study was approved by the Institutional ethics committee. The confidentiality of patients is respected and we consider that their anonymity can be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Pachychoroid neovasculopathy: Aspect on optical coherence tomography angiography. Acta Ophthalmol. 2017;95:421-7.
- [CrossRef] [PubMed] [Google Scholar]
- Spectrum of pachychoroid diseases. Int Ophthalmol. 2018;38:2239-46.
- [CrossRef] [PubMed] [Google Scholar]
- The choroid and optical coherence tomography. Turk J Ophthalmol. 2016;46:30-7.
- [CrossRef] [PubMed] [Google Scholar]
- Subfoveal choroidal thickness in fellow eyes of patients with central serous chorioretinopathy. Retina. 2011;31:1603-8.
- [CrossRef] [PubMed] [Google Scholar]
- Optical coherence tomography: Imaging of the choroid and beyond. Surv Ophthalmol. 2013;58:387-429.
- [CrossRef] [PubMed] [Google Scholar]
- Reproducibility of choroidal thickness measurements in healthy Turkish subjects. Eur J Ophthalmol. 2014;24:202-8.
- [CrossRef] [PubMed] [Google Scholar]
- Repeatability of manual subfoveal choroidal thickness measurements in healthy subjects using the technique of enhanced depth imaging optical coherence tomography. Invest Ophthalmol Vis Sci. 2011;52:2267-71.
- [CrossRef] [PubMed] [Google Scholar]
- Persistent and bilateral choroidal vascular abnormalities in central serous chorioretinopathy. Retina. 1999;19:508-12.
- [CrossRef] [PubMed] [Google Scholar]
- Optical coherence tomography characterisation of idiopathic central serous chorioretinopathy. Br J Ophthalmol. 2005;89:562-4.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of retinal and choroidal thickness by swept-source optical coherence tomography: Repeatability and assessment of artifacts. Am J Ophthalmol. 2014;157:1022-32.
- [CrossRef] [PubMed] [Google Scholar]
- Enhanced depth imaging optical coherence tomography of the choroid in central serous chorioretinopathy. Retina. 2009;29:1469-73.
- [CrossRef] [PubMed] [Google Scholar]






